The European Commission’s Vision for Pharma’s Future: Will Industry Get on Board?

The European Commission (EC) has set its sights on the most transformative reforms to pharmaceutical legislation in over two decades. The question is: Will the industry embrace these ambitious changes or push back?
Why Reform Now?
On April 26, 2023, the EC unveiled groundbreaking legislative proposals, marking the largest overhaul of the EU’s pharmaceutical legislation since 2004. This reform is driven through a comprehensive multi-stakeholder evaluation of its 2004 revisions. While the 2004 legislation has generally achieved its two primary goals (protecting public health and harmonizing the EU medicines market), the EC identified six major deficiencies that still need to be addressed:
| Deficiencies | Drivers | Impact |
| 1. Unmet Medical Needs of Patients | Insufficient incentives for small- and medium-sized enterprises (SMEs) to innovate, with high commercial risk for developing novel targeted treatments | Millions of patients lack effective treatments, especially for rare diseases |
| 2. Unequal Access to Medicines Across the EU | Disparities among Member States’ health technology assessment (HTA) and pricing and reimbursement (P&R) evaluation criteria lead to significant variations in launch timings (4 to 29+ months) | Medicines encounter entry barriers and launch delays or voluntary market withdrawals |
| 3. Affordability of Medicines | Expensive innovative medicines, combined with evergreening practices, delay the entry of generics and biosimilars | High drug costs strain underfunded health system budgets, leading to inequitable patient access (especially in Eastern and Southern Europe) |
| 4. Supply Shortages are Becoming More Frequent | Unprecedented medicine shortages during the COVID-19 pandemic highlighted lack of agility and urgency to enhance supply chain resiliency | Patients receive delayed or sub-optimal treatments |
| 5. Lack of Innovation Support and Administrative Burden | Rigidity, complexity and inefficiency of the current pharmaceutical framework | Unnecessary costs and time wasted for developers, weakening commercial opportunities |
| 6. Medicines in the Environment | Attempts to regulate drug-related pollution have proven ineffective | Pharmaceutical by-products pollute ecosystems, posing health risks |
The Path to Pharma Reform: Will It Work?
To increase the EU’s competitiveness and shift drug developers’ focus away from what has traditionally been a US-first commercialization approach, the EC must propose bold changes to the pharmaceutical framework. These reforms aim to enhance innovation, streamline access to life-saving drugs, and combat global health threats like antimicrobial resistance (AMR). The EC’s plans center on five key objectives.
The objectives set by the EC are ambitious, but will they be able to adequately address the deficiencies identified? The arguments presented by the EC are compelling, being backed by insights and extensive data gleaned from multi-stakeholder public consultations, retroactive analyses and budget impact assessments.
| Objectives | Goals | Prescient Perspectives |
| 1. Promote Innovation | Drive innovation in diseases with high unmet medical need as the primary focus, with a secondary focus on developing novel antimicrobials | The EC seeks to strengthen the EU’s R&D sector and boost the development of innovative medicines, noting that only 20% of authorized drugs originate in the EU. With proper incentives, the EC forecasts more innovative launches, including in addressing AMR through a first-of-its-kind transferable exclusivity voucher (TEV) scheme. |
| 2. Ensure Access and Enhance Supply | Promote timely and equal access while ensuring continuous supply and limiting shortages of medicines | Perhaps the most ambitious objective, with the EC aiming to make sweeping, long-term behavioral changes to both industry and public players in the spirit of a level playing field; ignoring certain markets or abusing negotiating practices may become a very expensive business decision for drug developers if the EC has its say. |
| 3. Create a Balanced System of Affordability and Innovation | Enable competition while promoting affordability and sustainable costs across the EU, with a focus on transparency | The EU hopes to see a surge of SMEs pursuing riskier innovative medicines, biosimilar developers being empowered to conduct more informed negotiations, and patients and healthcare providers making more informed decisions around treatment options. |
| 4. Reduce Regulatory Burden and Increase Flexibility | Simplify and integrate regulatory requirements by leveraging digital technology to reduce drug approval times and costs | Efforts to simplify, digitize and eliminate duplicative administrative processes will lead to faster time-to-market and greater commercial certainty for developers. |
| 5. Reduce Environmental Impact | Minimize medicine residues in the environment following their production, use and disposal | We expect tighter environmental regulations and standards, though the EC’s ability to broadly and effectively enforce these remains questionable. |
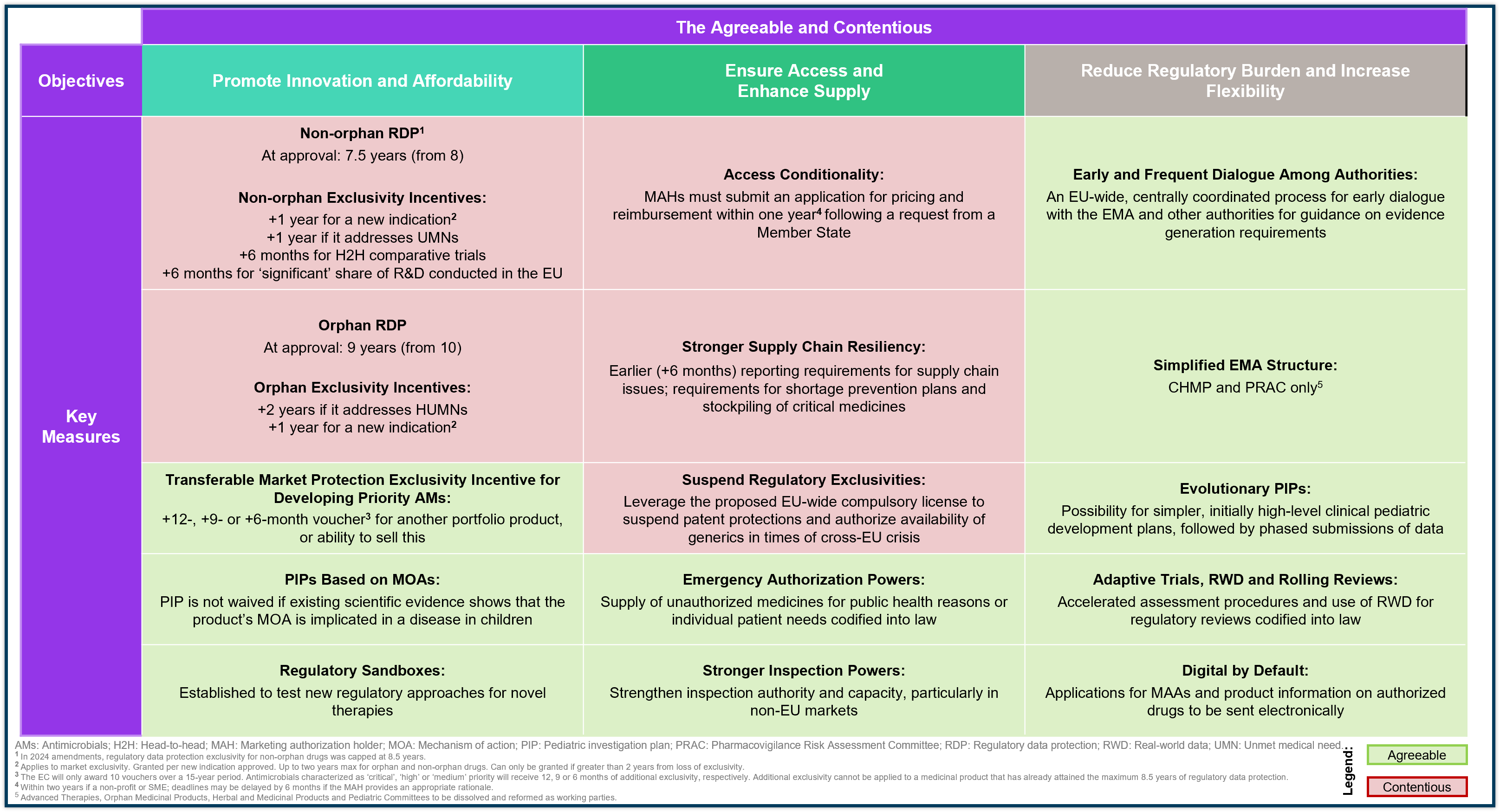
The Fine Print: Policies at the Heart of the Debate
These objectives, and their associated policy measures, look to strike a balance between incentivizing innovation while addressing unmet medical needs, enhancing access and affordability, and better responding to an increasingly unpredictable global health environment. The EC’s reform package includes over 70 policy measures and while some have been widely accepted, others have sparked intense debate.
April 2024 Update: The European Parliament overwhelmingly approved the reform package, introducing key amendments to the EC’s original 2023 draft. With this update, important amendments have been incorporated into the initial draft released in 2023. Key revisions include changes to regulatory data protection incentives, access conditionality requirements, and priority antimicrobial development incentives, among others. Overviews of some of more important amendments are as follows:
Agreeable Policies
Cutting Red Tape
The Push into the 21st Century: Improving the agility and navigability of the EMA’s regulatory framework
Key Points: The EC seeks to reduce administrative burdens and modernize EMA operations.
Prescient Perspectives: Streamlined processes will help drug developers achieve business predictability. A clearer, revamped pediatric investigation plan (PIP) framework reduces risk and uncertainty. R&D teams will benefit from increased scientific and regulatory support, allowing their clinical development plans to evolve with the pharmaceutical framework.
Tackling AMR
The Silent Pandemic: Incentivizing the development of novel antimicrobial treatments
Key Points: TEVs incentivize developers of priority antimicrobials with exclusivity for other products in their portfolio.
April 2024 Update:
- The TEV scheme now includes varying exclusivity durations based on antimicrobial priority level (based on WHO Priority Pathogens List)
- Developers cannot apply TEVs to products with maxed-out RDP (8.5 years)
- A milestone payment scheme and subscription-based procurement ensure developers receive fixed payments, delinking sales from usage
Prescient Perspectives: The TEV scheme could spur innovation in fighting “superbugs,” benefiting SMEs and developers with near-expiring blockbuster drugs. The potential to sell TEVs adds further value to developers.
Contentious Policies
Regulatory Data Protection
The ‘Quid Pro Quo’ Approach: Driving innovation for unmet medical needs (UMNs)
Key Points: Baseline RDP for non-orphan drugs reduced from 8 to 7.5 years, with a max of 8.5 years. Orphan drug RDP reduced from 10 to 9 years. Developers can gain back exclusivity by meeting criteria like addressing UMNs or conducting head-to-head trials.
April 2024 Update: Baseline RDPs further adjusted for non-orphan drugs, with additional RDP incentives now provided for UMN-targeting orphan drugs.
Prescient Perspectives: This strategy encourages innovation by offering incentives to regain RDP, focusing on rewarding the development of therapies for UMNs.
Access Conditionality
The Carrot and Stick Model: Achieving equal access to medicines for all
Key Points: Developers had two years (three for orphan drugs) to secure P&R approval in all 27 Member States or face earlier generic/biosimilar competition.
April 2024 Update:
- The two-year data protection extension for launching in all EU Member States has been removed
- Member States now formally request P&R approval, with marketing authorization holders (MAHs) required to respond within a year
- SMEs get two-year deadlines to respond, with potential extensions for all developers
- MAHs must ensure continuous supply once P&R is approved
Prescient Perspectives: Initially, the access conditionality was highly contentious. Following significant industry pushback, the EC has since shifted the responsibility for initiating P&R negotiations to Member States, addressing concerns about the varied capacities of national HTAs and harmonization challenges within the EU.
What’s Next?
Although the reforms have passed a key legislative hurdle, the journey is far from over. With a new EU Parliament sworn in on July 16, 2024, and ongoing industry negotiations, these proposals could still see significant changes. The next draft isn’t expected until 2025, with adoption coming in late 2025 or early 2026, and full implementation not expected until 2027-2028 due to a mandatory 18-month holding period for Member States.
Pharma companies must pay close attention to these developments. Cross-functional collaboration between R&D, regulatory and market access teams will be critical to staying ahead in this dynamic landscape. The EC has signaled a willingness to adapt its policies, so the industry must remain actively engaged in negotiations. Change is coming, and it’s time to be ready.
Call to Action: How Prescient Can Support You Through Change
At Prescient, we understand the impact policies and regulations can have on your R&D and commercial strategies. With our deep expertise in market access and competitive strategy and market-specific knowledge, we are uniquely positioned to help you navigate complex regulatory landscapes. By leveraging insights from industry peers, we enhance your market positioning and better allow you to foresee future challenges.
Whether you’re looking to de-risk your clinical pipeline, develop tailored market access, or assess regulatory impacts on your portfolio, our team are here to guide you. Contact us today to stay ahead in the EU’s evolving pharmaceutical landscape.
References
- Press release: European Health Union: Commission proposes pharmaceuticals reform for more accessible, affordable and innovative medicines: https://ec.europa.eu/commission/presscorner/detail/en/ip_23_1843
- Frequently Asked Questions: Revision of the Pharmaceutical legislation: https://ec.europa.eu/commission/presscorner/detail/en/qanda_23_1844
- Proposal for a Directive of the European Parliament and of the Council on the Union code relating to medicinal products for human use, and repealing Directive 2001/83/EC and Directive 2009/35/EC: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52023PC0192
- Proposal for a Regulation Of The European Parliament and of the Council laying down Union procedures for the authorisation and supervision of medicinal products for human use and establishing rules governing the European Medicines Agency, amending Regulation (EC) No 1394/2007 and Regulation (EU) No 536/2014 and repealing Regulation (EC) No 726/2004, Regulation (EC) No 141/2000 and Regulation (EC) No 1901/2006: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52023PC0193
- Commission Staff Working Document Impact Assessment Report: https://health.ec.europa.eu/system/files/2023-04/swd_2023_192_1_ia_en.pdf
- Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on health technology assessment and amending Directive 2011/24/EU: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32021R2282
- Compromise amendments to the proposed regulation: https://www.europarl.europa.eu/meetdocs/2014_2019/plmrep/COMMITTEES/ENVI/AMC/2024/03-19/Pharma_Regulation_Final_CAs_EN.pdf
- Compromise amendments to the proposed directive: https://www.europarl.europa.eu/meetdocs/2014_2019/plmrep/COMMITTEES/ENVI/AMC/2024/03-19/Pharma_Directive_Final_CA_EN.pdf
- Pharmaceutical package: extracts from adopted ENVI reports: https://www.europarl.europa.eu/news/en/press-room/20240318IPR19418/pharmaceutical-package-extracts-from-adopted-envi-reports
- WHO Priority Pathogens List: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed