Metastatic Castration-Resistant Prostate Cancer: A Closer Look at Emerging Therapies to Watch
Jan 22, 2024

- 1. Prostate cancer and its challenges:
Prostate cancer ranks as the second leading cause of cancer-related deaths in the US, with approximately 34,700 fatalities due to this disease in 2023. Further, this is also the most commonly diagnosed cancer among men, with a predicted incidence of 288,300 cases in the same year1. Prostate cancer usually begins as a slow growing, immunologically cold, solid tumor. While, in the majority of cases, the cancer initially responds well to androgen-deprivation therapy (ADT), as it progresses, it has the potential to metastasize and develop resistance, leading to metastatic castration-resistant prostate cancer (mCRPC). mCRPC is characterized by clinical, radiographic or biochemical progression despite treatment with ADT. In the US, there were approximately 98,000 cases of mCRPC in 2022 and the prevalence is expected to rise approximately 10% over the next 5 years2, which presents a tremendous challenge for patients and healthcare providers (HCPs). Despite the approval of novel compounds, mCRPC still yields poor outcomes, being associated with a median OS of approximately 1-3 years, varying on the location of metastasis3,4.
- 2. Evolution of the treatment landscape:
The treatment options for mCRPC have historically been limited, primarily featuring chemotherapy and palliative care. The first breakthrough in mCRPC treatment occurred in 2004 when docetaxel chemotherapy was approved by the FDA, driven by its OS benefit5. Docetaxel is effective as a first-line (1L) option for rapidly progressing mCRPC and in cases with visceral metastases. This approval marked a shift in the mCRPC treatment paradigm from palliative care to therapeutic care that prolongs survival. Following docetaxel’s approval, another taxane-based treatment, cabazitaxel, was approved in 2010. Over the subsequent three years, the treatment paradigm expanded beyond chemotherapy, offering more options for patients and prescribers, as shown in Figure 1. Androgen receptor pathway inhibitors (ARPI), also known as novel hormonal therapy, and additional options for specific subpopulations, such as sipuleucel-T and Radium-223, were introduced during this period. The last few years saw a third wave of innovation, with the approval of poly(ADP-ribose) polymerase inhibitors (PARPi) and PSMA-targeting radiopharmaceuticals.
Figure 1. Approval Milestones for Advanced Prostate Cancer
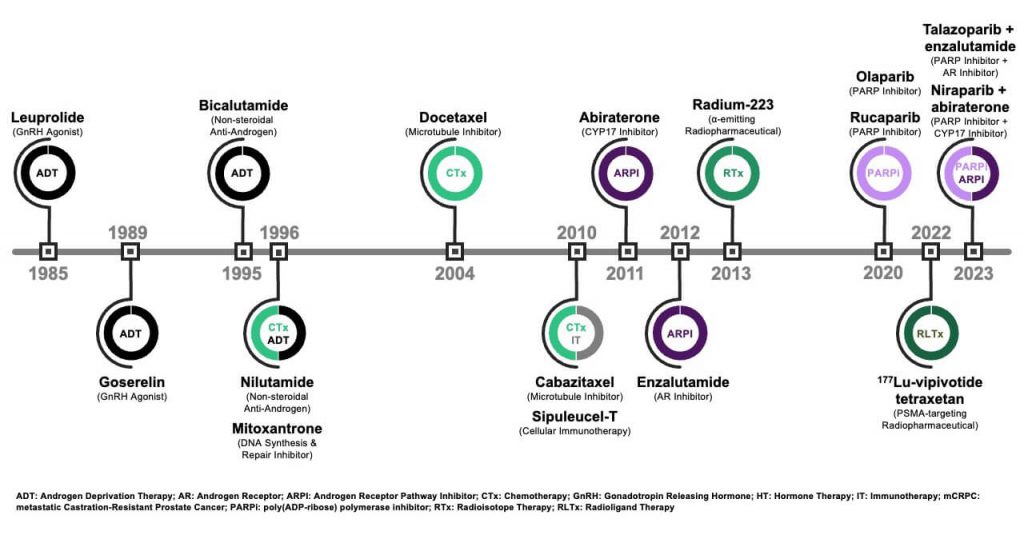
Current 1L options6 for mCRPC include ADT in combination with an ARPI, such as abiraterone, a CYP17 inhibitor that interferes with intra-tumoral androgen synthesis, or enzalutamide, an androgen receptor (AR) antagonist. Both agents have comparable efficacy and are prescribed based on AE tolerability, physician preference and payer-specific considerations7,8. Docetaxel also remains as a 1L option. The PARPi olaparib9 and niraparib10 can be used in combination with abiraterone for patients with breast cancer gene (BRCA) mutations, while talazoparib in combination with enzalutamide is also an option for patients with mutations in other homologous recombination repair (HRR) genes11.
The treatment options in second-line (2L) focus on changing mechanisms of action (MoA) from 1L, depending on the initial therapy (e.g., ARPI is followed by chemotherapy). For patients who have been previously treated with both ARPI and taxane-based chemotherapy, lutetium (177Lu)-vipivotide tetraxetan, a prostate-specific membrane antigen (PSMA)-targeting radioligand agent, can be used as a treatment for PSMA-positive mCRPC12. Further options that have demonstrated notable OS benefits include sipuleucel-T, an autologous vaccine immunotherapy for asymptomatic/minimally symptomatic mCRPC13, and radium-223, an α-emitting radioisotope for mCRPC cases with bone metastases.
- 3. Recent mCRPC pipeline updates:
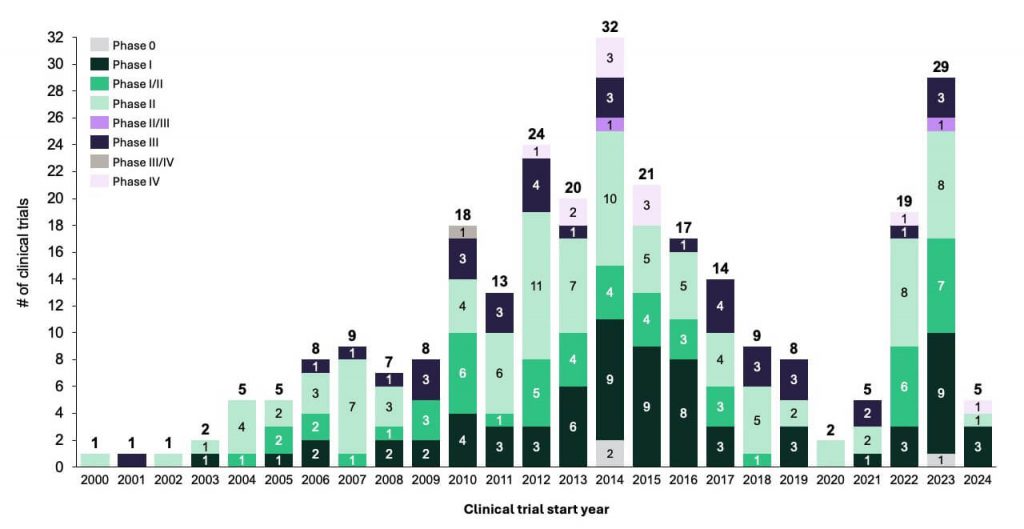
While a curative agent has not been developed, there is an ongoing effort to develop new life-extending treatments for mCRPC, stemming from the significant unmet needs in this area. Recent successes and the identification of options with novel MoA have led to a strong increase in trial activity (Figure 2). The primary focus is on pursuing novel treatments that minimize cross-resistance, improve survival outcomes and optimize therapy sequencing to maximize patient benefits. In the following sections, we will explore the growing pipeline of agents, examining their MoA, ongoing clinical trials and the potential impact on the evolving landscape of mCRPC.
Figure 2. Clinical Trials in mCRPC
- a) Novel radiopharmaceuticals changing the treatment paradigm for mCRPC
Radioligand therapy is becoming a crucial element for prostate cancer treatment. In March 2022, the FDA approved 177Lu-vipivotide tetraxetan (Pluvicto™) as a 2L treatment for PSMA-positive mCRPC, following treatment with ARPIs and taxane. This approval was based on the favorable results from the Phase III VISION trial (NCT03511664) comparing 177Lu-vipivotide tetraxetan to the best standard of care (BSoC), which showed that the former led to improved radiographic progression-free survival (rPFS) and OS than the latter. Several clinical trials are underway to assess this asset’s efficacy in earlier lines of therapy. The interim results from the Phase III PSMAfore trial (NCT04689828), conducted in the earlier pre-taxane mCRPC setting, were presented at ESMO 202314. The results showed that 177Lu-vipivotide tetraxetan achieved the rPFS primary endpoint (12.02 months with 177Lu-vipivotide tetraxetan compared to 5.59 months in the ARPI control arm). Furthermore, this asset has demonstrated a remarkable ten-fold increase in complete response rate (21.2% vs. 2.7%) vs. the comparator arm. Further data from PSMAfore, including OS, will guide the FDA filing planned for 2024. Expansion plans into earlier lines are underway, contingent on the data readout from the PSMAddition trial (NCT04720157).
A similar agent, 177Lu-PNT2002, recently received Fast Track Designation from the FDA15. This agent is being investigated in a Phase III trial (NCT04647526) as treatment for PSMA-positive mCRPC patients that progressed after ARPI therapy and refuse or are ineligible for chemotherapy. The trial readout is anticipated in 2024. Additionally, 177Lu-PSMA-I&T, another radioligand, is under investigation both as a standalone treatment (NCT05204927) and in combination with 223Ra (NCT05383079) for mCRPC16. The wave of momentum for radioligand therapy in prostate cancer seems poised to persist into 2024.
- b) Emerging MoA in mCRPC
Ongoing research is exploring the viability of alternative treatment modalities for mCRPC. These include kinase inhibitors (e.g., abemaciclib and capivasertib), bromodomain extra-terminal (BET) inhibitors (e.g., ZEN-3694) and AR-degrading agents (e.g., bavdegalutamide). Therapies seeking to increase the immunogenicity of tumors, such as bispecific antibodies and chimeric antigen receptor T-cell therapies (CAR-T), are also being actively explored.
Proteolysis-targeting chimera (PROTAC) AR degraders, such as the 1st generation bavdegalutamide, recently garnered attention as a potential option for a subset of mCRPC patients who carry AR ligand binding domain (LBD) mutations, who constitute approximately 10-20% of all mCRPC patients. Patients with these mutations often face a poor prognosis, as the frequency of the mutations increases with ARPI treatment and is thought to be linked to treatment resistance17. The Phase I/II trial (NCT03888612) evaluating bavdegalutamide acted as a proof-of-concept for the notable clinical efficacy of this asset in patients with AR LBD mutations; new data shared at ESMO 2023 showed a median rPFS of 8.2 months (95% CI: 3.8–not reached) with this drug in AR LBD mutant patients18. The 2nd generation AR degrader ARV-766, however, exhibited higher efficacy compared to its predecessor. PSA50 was achieved in 41% of all LBD patients receiving ARV-766 in a Phase I/II trial (NCT05067140), according to the interim results. Furthermore, ARV-766 exhibited a better safety and tolerability profile. These results, combined with ARV-766’s estimated potential to benefit a larger group of patients with mCRPC and mCSPC, led to it being prioritized for Phase III progression in mCRPC and mCSPC19.
Other notable mentions include antibody-drug conjugates (ADC) in development for mCRPC. HS-20093, a fully humanized IgG1 that selectively binds to B7-H3, a target that is abundantly expressed on solid tumor cells, had an acceptable safety profile and showed promising antitumor activity, as demonstrated by the partial response to therapy observed in the Phase I ARTEMIS-001 trial (NCT05276609). This led the asset to advance into a Phase II clinical trial (NCT05276609).
- c) Novel combinations
The exploration of novel combinations of agents with complementary MoAs has been another promising strategy for the treatment of mCRPC. One notable combination to watch for in 2024 is cabozantinib, a tyrosine kinase inhibitor (TKI), plus atezolizumab, an immune checkpoint inhibitor. This combination has already achieved the primary endpoint of PFS in the pivotal Phase III CONTACT-02 trial (NCT04446117)20. Additionally, a positive trend for OS improvement was observed with the cabozantinib and atezolizumab combination compared to a second combination with ARPIs (e.g., abiraterone plus prednisone or enzalutamide) in patients with previously treated mCRPC. Definitive conclusions, however, cannot be drawn until OS data maturity is reached in H2 2024.
Additional studies evaluating combinations to monitor include a variety of trials exploring radioligand combinations21. Noteworthy among them is the Phase II EVOLUTION trial (NCT05150236), investigating the safety and efficacy of 177Lu-PSMA-617 combined with ipilimumab and nivolumab, immune checkpoint inhibitors, with an estimated completion in H2 2024. Additionally, the Phase III ProsTACT trial (NCT04876651) compares the combination of 177Lu-rosopatamab tetraxetan and an HCP’s preferred BSoC, such as taxane or ARPI, against BSoC monotherapy. ProsTACT, initiated in November 2023, is projected to conclude in Q4 202722.
- 4. Utility of genomic profiling in treatment selection
Understanding the genomics of prostate cancer is crucial to tailor effective treatment strategies, particularly for mCRPC patients. Genomic testing, encompassing germline and somatic assessments, plays a pivotal role. Additionally, RB, PTEN or p53 mutations may not only impact treatment decisions but also carry a substantial prognostic and predictive value23. The recent approvals of PARPi highlight the need to routinely identify mutated signaling pathways, as DNA repair mutations are present in approximately 23% of mCRPC cases.
There has been a surge in routine mCRPC genetic testing and a consensus in recommending integrating cancer genetics into the care plan. There are several implementational challenges, such as the rising demand for genetic counseling and testing, combined with existing accessibility barriers (e.g., geographic constraints on in-person services), which have created bottlenecks in testing. While considerable progress has been made, the routine application of genomics in mCRPC globally is still evolving24. Ongoing trials aim to strengthen the routine application of genetic testing as a standard for patients25, while research for treatments targeting other pathways, as described above, will further increase the need for testing over the coming years.
- 5. Outlook
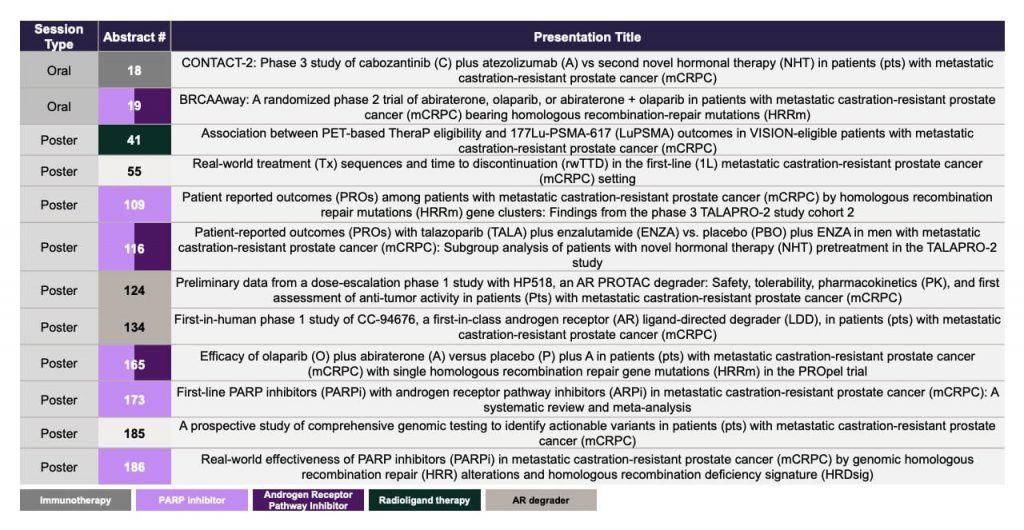
The evolving landscape of mCRPC treatment, which is driven by ongoing research and innovative approaches, offers the potential for a deeper understanding of the molecular intricacies of advanced prostate cancer and novel treatment options for patients. The increased research activity can be expected to produce a wealth of new data over the coming years. Updates on novel treatment options, treatment sequencing, biomarkers and genetic testing will be presented at the upcoming American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU) on January 25-27 202426, with a selection of noteworthy abstracts highlighted in Figure 3. The symposium is positioned to enhance our understanding of prostate cancer and provide an outlook into the future of the treatment options for mCRPC.
Figure 3. Abstracts to watch – ASCO GU 2024
References
1 Cancer statistics, 2023. Ca Cancer J Clin, 73(1), 17-48 (2023).
2 GlobalData Epidemiology and Market Size of mCRPC. [Accessed 21 December 2023]
6 NCCN guidelines, Prostate Cancer, Version 4 (2023).
9 FDA approves olaparib with abiraterone and prednisone (or prednisolone) for BRCA-mutated metastatic castration-resistant prostate cancer. [Accessed 22 December 2023]
10 FDA approves niraparib and abiraterone acetate plus prednisone for BRCA-mutated metastatic castration-resistant prostate cancer. [Accessed 22 December 2023]
11 FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. [Accessed 22 December 2023]
18 Potential of Arvinas’ PROTAC® AR Degraders Reinforced by 11.1 months rPFS with Bavdegalutamide and Updated Positive Interim Data from Second Generation ARV-766 in mCRPC. [Accessed 22 December 2023]
19 ARVINAS AR Franchise Update – Bavdegalutamide and ARV-766 (2023).
22 First Patient Dosed in Phase III ProstACT GLOBAL Study of Antibody-based Prostate Cancer Therapy Candidate, TLX591 (2023). [Accessed 16 January 2024]
23 Prognostic value of genomic mutations in metastatic prostate cancer. Heliyon, 9(3) (2023).
25 Genomics of prostate cancer: clinical utility and challenges. Acta Clin Croat. 61(Suppl 3):86 (2022).
